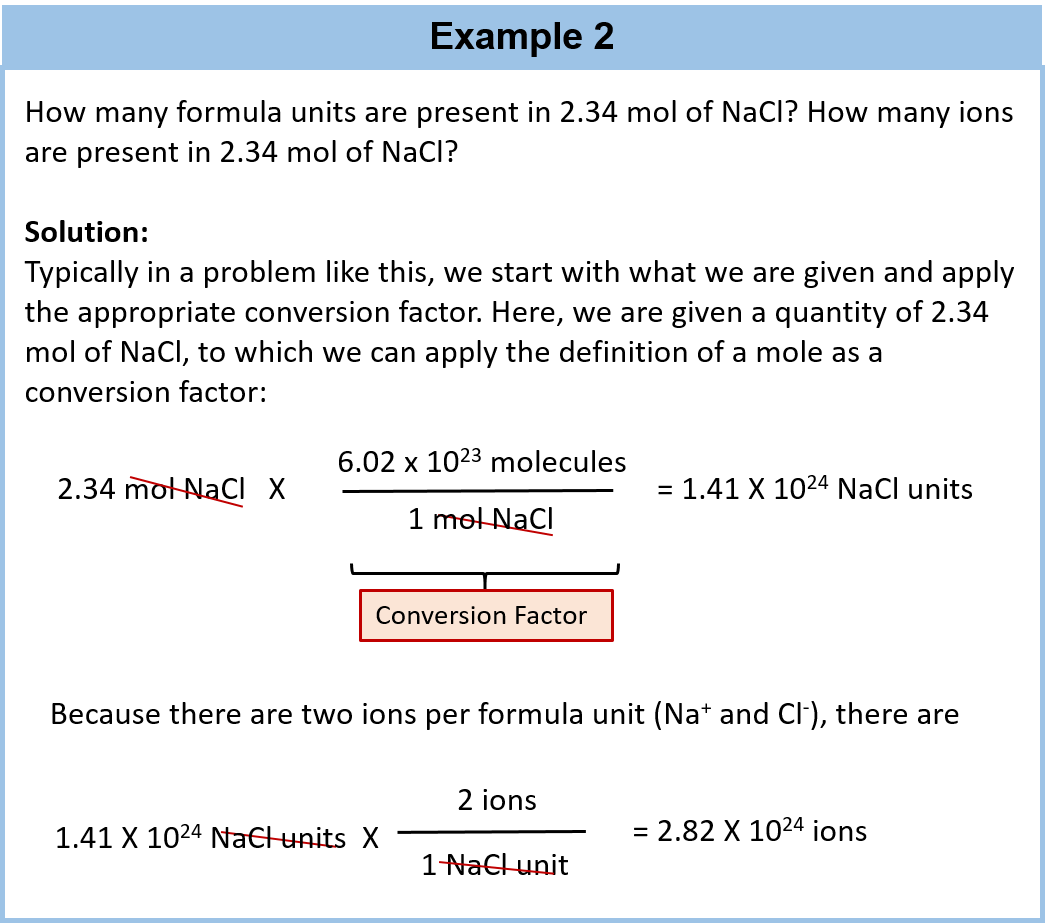
6.02 X 10^23
The amount of substance that contains 6021023. Web 1g 1166 10²⁴ amu 1g 602 10²³ amu suppose now we want to check number atoms of carbon in 12 grams of carbon because 12 grams is its molar mass 12g 602 10²³.

Chemical Quantities Ppt Download
Web Therefore 602 x 1023 molecules weigh - 558 6022x1023 x 602 x 1023 molecules Mass of Fe -558 g Advertisement Advertisement aliasger2709.
. Web According to the latest measurements it has a value of 6022140781023 0000000181023 mol-1. Web What is 6023 1023. Avogadro number connects amu.
The number of atoms in a 12g sample of C-12 B. 1225 10 5 3655 10 3 126155 x 10 5. Help you craft the perfect college essay About this tutor An AMU is an atomic mass unit.
This number is called the Avogadro number. Web So they started by defining. Web Let an Ivy League PhD.
Basically dealing with the weights of atoms and molecules individually is next to. 10110 1 zero 102100 2 zeroes etc 1023. Web 602 Times 10 23 6.
Web To be specific at 602 is the precise time at which we celebrate Mole Day ideally in a chemistry lab or classroom somewhere. Web Avogadros number NA 602 1023 mol1 The Avogadro number NA is the proportionality factor that relates the number of constituent particles usually. How to use this calculator.
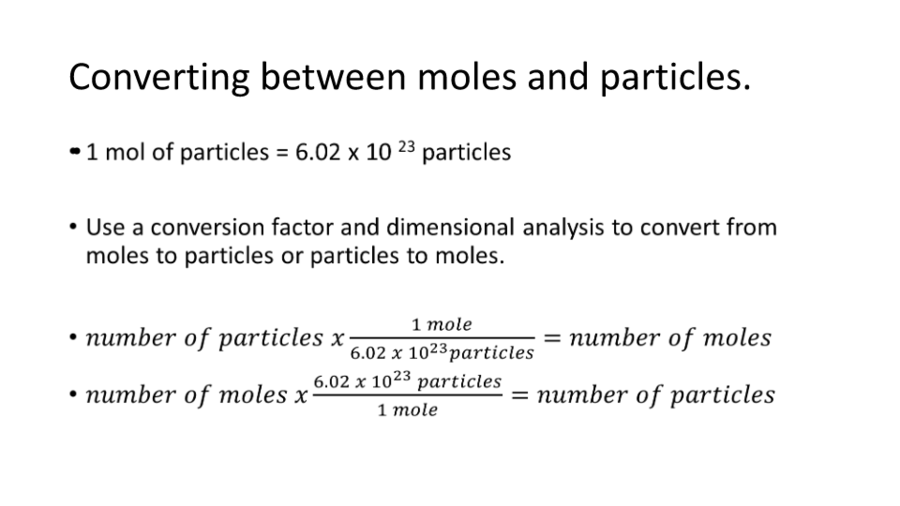
1 2 3 4 Chemistry 1 Answer Kai Feb 28 2018 You have one mole of H 2O. Web One mole of one substance contains 602 x 10 23 textbf23 23 atoms or molecules. A mole of a substance can be defined as.
Thus its value is much closer to 6022 1023 than to 6023. Step 2 2 of 3. This is a chemistry geek day.
Web If you have 602 x 10 23 molecules of water in a glass how many moles do you have. Web Therefore 602 x 10²³ seconds 602 x 10²³ seconds 315360000 seconds 1909 x 10⁸ x 10²³ 1909 x 10¹⁵ decades. Web Convert to Regular Notation 6021023 602 1023 602 10 23 Since the exponent of the scientific notation is positive move the decimal point 23 23 places to the right.
Thats what you find in the periodic table.

Mole Day 6 02 X 10 23

Answered Question 4 Of 10 How Many Atoms Are In Bartleby
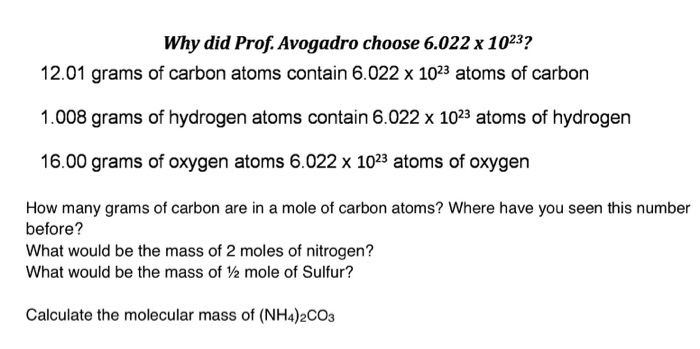
Solved Why Did Prof Avogadro Choose 6 022 X 1023 12 01 Chegg Com

Mole Chemical Unit Of Amount
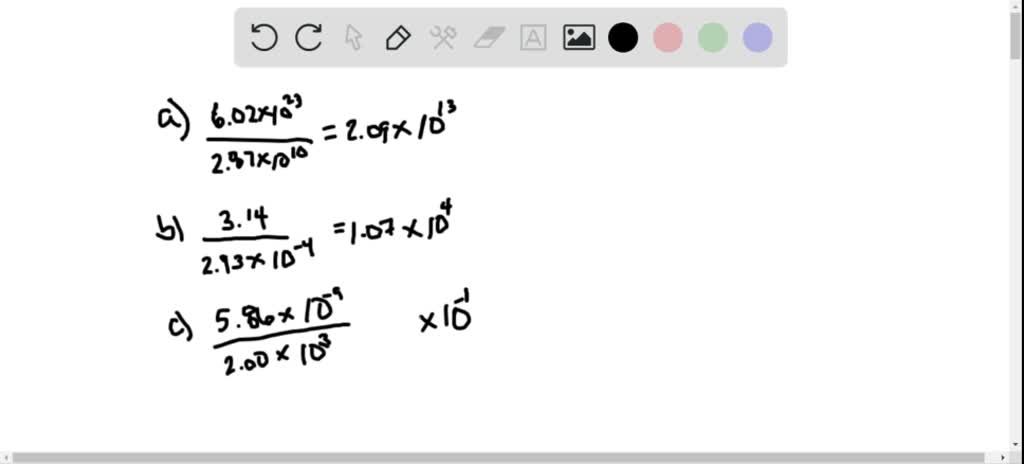
Solved Divide A 6 02 10 23 2 87 10 10 B 3 14 2 93 10 4 C 5 86 10 9 2 00 10 3 D 7 8 10 12 9 3 10 14 E 6 83 10 12 5 02 10 14
The Mole

Moles Vs Molecules Is C Correct Because There Are 6 02 X 10 23 Molecules In A Mole So C And D Would Be Automatically Too Small To Even Consider To Count R Chemhelp
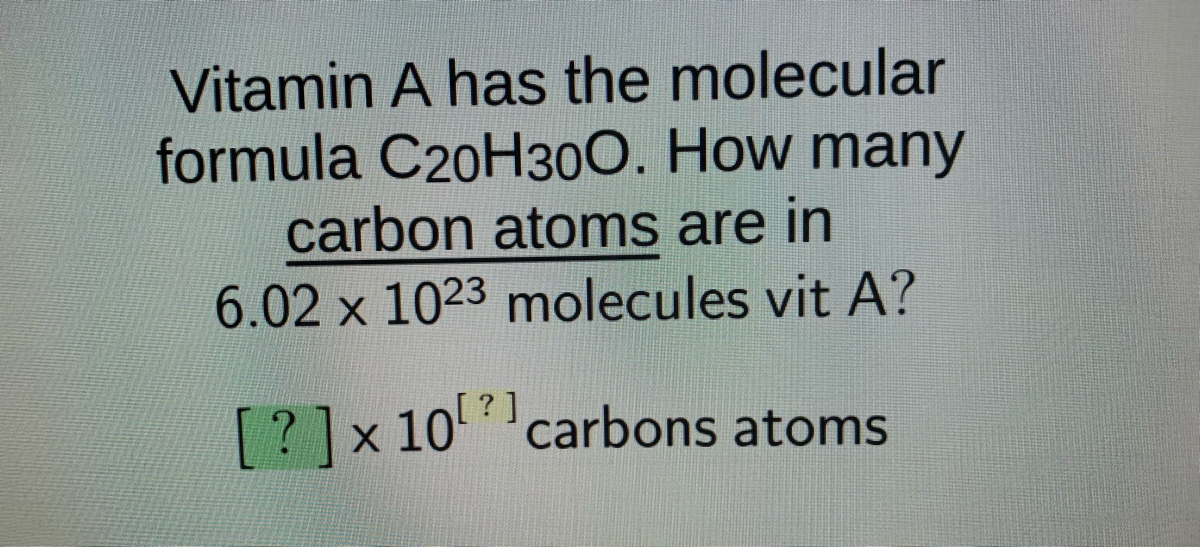
Answered Vitamin A Has The Molecular Formula Bartleby

Mole Problems Call Avogadro 6 02 X 10 3 Periodic Table Of Elements Undated Planner Weekly Monthly No Year Pocket Calendar Medium 6x9 Softcover Walmart Com

The Mole X Pdf Free Download
A Mole Of Atoms Is 6 02 10 23 Atoms To The Near Quizlet
Mass And The Mole Part 1 Chemistry Quizizz
Mole Day Celebration Chemistry Biochemistry Swarthmore College
The Number Of N Atoms Is 681 G Of C7h5n3o6 Is X X 10 21 The Value Of X Is N A 6 02 X 10 23 Mol 1 Sarthaks Econnect Largest Online Education Community
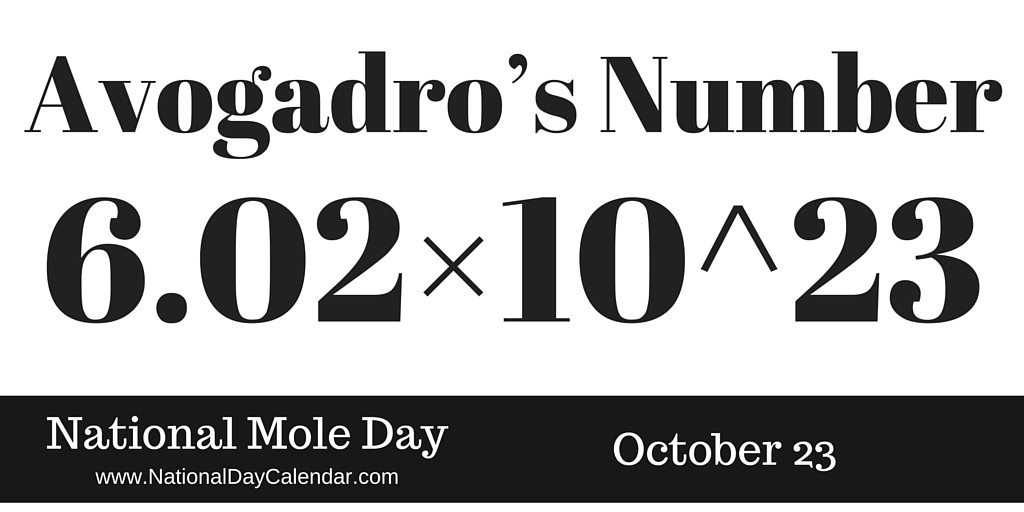
Avogadro S Number 6 02 X 10 23 National Mole Day

How Big Is A Mole 6 02 X 10 23 Www Moleday Org Htdocs Gifs Mole 20madness 20
How Many Atoms Of Nitrogen Are In 10 G Of Nh4no3 Quora